Celltrion's Zymfentra to make US debut in February
By Shim Woo-hyunPublished : Nov. 30, 2023 - 16:56
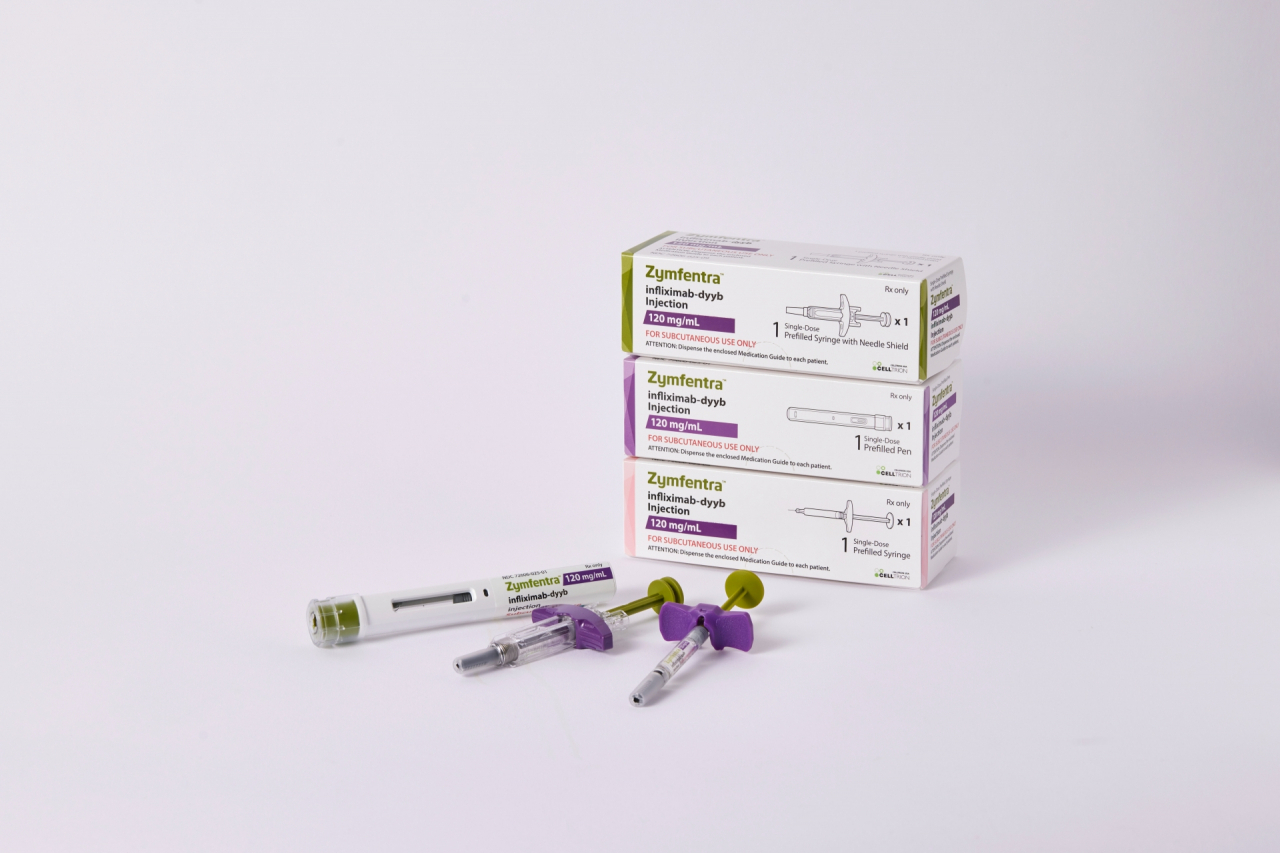
Celltrion Healthcare, Celltrion Group's sales arm, announced Thursday that the company’s autoimmune disease treatment Zymfentra will make its debut in the US market on Feb. 29 next year.
Zymfentra, which is also known as Remsima SC, is a subcutaneous injection formulation of Celltrion's infliximab Remsima, a treatment for patients with rheumatoid arthritis, Crohn’s disease, ulcerative colitis, ankylosing spondylitis, psoriatic arthritis and psoriasis in adult patients.
Zymfentra won approval from the US Food and Drug Administration on Oct. 20, and Celltrion Healthcare is speeding up the US launch, according to the company.
“Celltrion Healthcare has submitted clinical research data to a number of pharmacy benefit managers (PBMs) and is currently in discussions with some of them,” an official from the company said. “The company expects that it can successfully enlist Zymfentra in some of their formulary lists by the time when it launches the treatment next year.”
PBMs are firms that manage prescription drug benefits on behalf of health insurers. They negotiate fees and rebates with drug providers and create formulary lists of medications covered by insurance and reimburse pharmacies for patients' prescriptions.
Celltrion Healthcare said the company will soon begin marketing activities toward doctors in the US, particularly those who specialize in autoimmune diseases, to encourage prescriptions of Zymfentra.
The company said it will join a number of gatherings of physicians and researchers in the US, including Crohn’s & Colitis Congress in January, Digestive Disease Week in May, American College of Gastroenterology in October and American College of Rheumatology in November.
Celltrion Healthcare will also double the number of sales representatives and marketing employees for Zymfentra at its US office to strengthen its sales capacity.
Zymfentra is one of Celltrion’s treatments that it expects can become a cash cow in the following years. Celltrion Group expects annual sales of Zymfentra to reach 3 trillion won ($2.3 billion) in three years.
Earlier in October, Celltrion Group Chairman Seo Jung-jin also said sales of Zymfentra could reach 5 trillion and go beyond that in the future.
Celltrion Group expects Zymfentra sales will also play an important role in the group’s long-term strategy to achieve an annual sales target of 12 trillion won by 2030.



















![[Today’s K-pop] Treasure to publish magazine for debut anniversary](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/07/26/20240726050551_0.jpg&u=)