Celltrion’s Remsima SC gets EMA approval, plans to launch in 2020
By Lim Jeong-yeoPublished : Nov. 26, 2019 - 14:47
Celltrion said Tuesday its infliximab biobetter Remsima SC has received the European Medicines Agency’s approval for sales in the continent.
Remsima SC’s main target patients are those who are resistant to original TNF-a inhibitors such as Humira (adalimumab) and Enbrel (etanercept).
Remsima SC’s main target patients are those who are resistant to original TNF-a inhibitors such as Humira (adalimumab) and Enbrel (etanercept).
While infliximab currently takes up only 9 percent of the rheumatoid arthritis treatment market, the rate of patients who are prescribed a different medication -- apart from adalimumab and etanercept -- due to resistance is over 25 percent every year, Celltrion said.
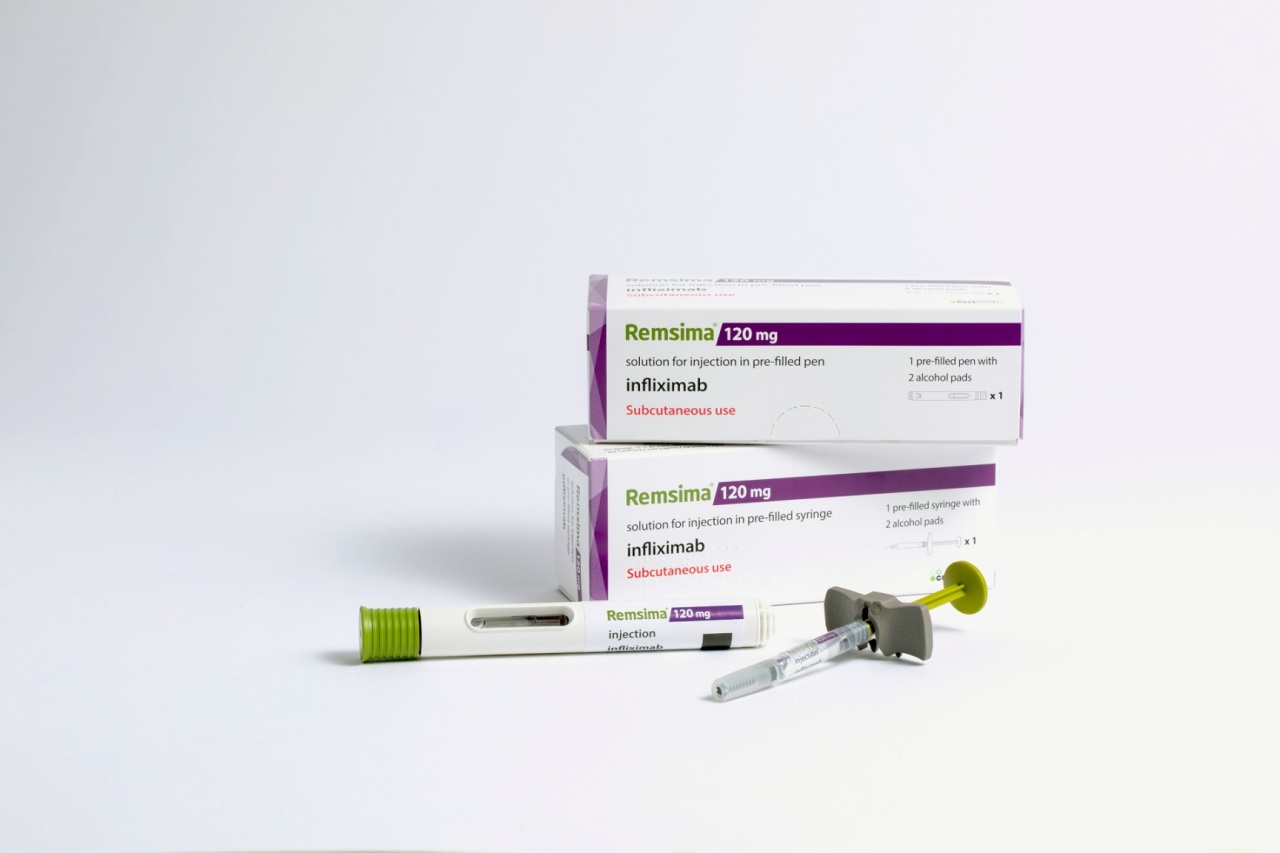
Remsima SC is the world’s first subcutaneously injectible infliximab that differentiates itself from the previous intravenously injectable formulation.
This enhanced administration convenience serves as a competitive edge for the autoimmune disease treatment, and makes Remsima SC a biobetter, not a biosimilar. Being a biobetter gives Remsima SC a longer patent time of 20 years, and allows the company to carry out clinical trials for each indication of the referenced Remicade.
In addition to the rheumatoid arthritis indication, Celltrion plans to clinically prove Remsima SC’s efficacy for inflammatory bowel disease, with the aim of seeking approval for it in mid-2020 and getting the green light for the total infliximab treatment scope.
Celltrion said it hopes to take up 90 percent of the infliximab market by end-2020.
Remsima SC will launch in major markets such as Germany, the UK and the Netherlands in the first quarter of 2020, estimated to be worth $4 billion. By the fourth quarter, the domination rate is expected to reach 89 percent, worth $7.9 billion, Celltrion projected.
The entire TNF-a inhibitor market size is estimated to be around $45.3 billion, combining that of infliximab, adalimumab and etanercept. RA takes up 63 percent of that market and IBD 27 percent.
Celltrion Healthcare, a subsidiary of Celltrion, will oversee the direct sales of Remsima SC in Europe in 14 nations.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)









![[Hello India] Hyundai Motor vows to boost 'clean mobility' in India](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/25/20240425050672_0.jpg&u=)








