As largest IPO of year, SK Biopharmaceuticals makes strong market debut
Mega-blockbuster IPO likely to empower SK Holdings, may speed up governance change
By Jie Ye-eunPublished : July 2, 2020 - 15:49

In the largest market debut of the year, SK Biopharmaceuticals was listed on the nation’s main bourse Kospi on Thursday, with its share price soaring to a daily limit in the first trading session.
Buoyed by the growing market interest in the bio industry as well as increased capital flow to the local stock market, the initial public offering of the SK subsidiary marked the highest offering deal since the 1 trillion-won market debut of Celltrion Healthcare in 2017.
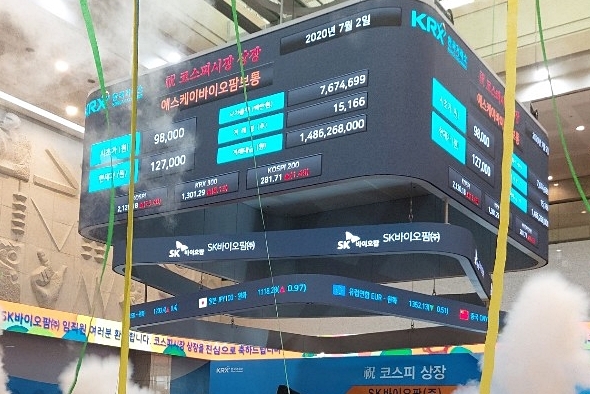
SK Biopharmaceuticals shares began trading at 98,000 won ($81.65), nearly double its offering price of 49,000 won. According to the nation’s sole stock bourse operator Korea Exchange, the opening price of a newly listed stock is determined between 8:30 a.m. and 9 a.m. in the first trading session and it can reach up to 200 percent of its offering price.
The stock price of the drug developer then spiked to the daily permissible limit of 30 percent to 127,000 won in early trading hours. It continuously maintained the price throughout the day, while marking its market capitalization of nearly 9.95 trillion won, becoming the 27th most-valued stock on Kospi at the closing bell.
“It feels like I am still dreaming. I’m so honored to list our firm on the stock market. All the hardship I had to go through seems to be forgotten,” said SK Biopharmaceuticals CEO Cho Jeong-woo. “Through such a historic moment (of the listing), we will put a lot of effort to accelerate our vision to rise as a global pharma firm.”
Buoyed by the growing market interest in the bio industry as well as increased capital flow to the local stock market, the initial public offering of the SK subsidiary marked the highest offering deal since the 1 trillion-won market debut of Celltrion Healthcare in 2017.
SK Biopharmaceuticals shares began trading at 98,000 won ($81.65), nearly double its offering price of 49,000 won. According to the nation’s sole stock bourse operator Korea Exchange, the opening price of a newly listed stock is determined between 8:30 a.m. and 9 a.m. in the first trading session and it can reach up to 200 percent of its offering price.
The stock price of the drug developer then spiked to the daily permissible limit of 30 percent to 127,000 won in early trading hours. It continuously maintained the price throughout the day, while marking its market capitalization of nearly 9.95 trillion won, becoming the 27th most-valued stock on Kospi at the closing bell.
“It feels like I am still dreaming. I’m so honored to list our firm on the stock market. All the hardship I had to go through seems to be forgotten,” said SK Biopharmaceuticals CEO Cho Jeong-woo. “Through such a historic moment (of the listing), we will put a lot of effort to accelerate our vision to rise as a global pharma firm.”
Prior to its market debut, local and offshore investors rushed for SK Biopharmaceuticals shares due to its growth potential amid the coronavirus pandemic. The retail investors’ tranche marked the end of its IPO to raise 959.3 billion won through 19,578,310 common shares.
In the two-day retail tranche that was held on June 23-24, the shares were oversubscribed 323 times with deposit payments amounting to 30.98 trillion won. The figure was the highest since Cheil Industries’ IPO in 2014 that garnered 30.06 trillion won in deposit payments.
The firm was looking to issue 13.3 million new shares and existing 6.3 million shares owned by parent SK Holdings up for fundraising via the IPO. Under the plan, the drug developer aims to enhance its global expansion by ramping up the cenobamate marketing and production.
It will add fuel to research and development efforts of its commercial and clinical-stage drugs to cure epilipsy, schizophrenia, attention deficit hyperactivity disorder and rare neurological diseases, in its bid to address unmet medical needs in the US, according to the officials.
SK Biopharmaceuticals, established 2011, is an innovative chemical drug maker whose focus is on treating disorders of central nervous system.
All of SK Biopharmaceuticals’ pipelines are for various CNS disorders which is both the company‘s bane and boon.
CNS disorders, because of their close ties to the brain, are difficult to cure. Treatments take years to develop and can always fail mid-attempt.
But this risk lurks in all drug development projects.
CNS drugs also have high unmet medical needs whose successful developments can crack the global market the size of $84 billion.
Global CNS drug market is anticipated to grow at an annual average rate of 6 percent, reaching $118 billion by 2024, SK Biopharmaceuticals said.
SK Biopharmaceuticals already has two CNS drugs authorized by the US’ Food and Drug Administration, the most austere standard-keeper in the global medicines screening field.
The first is solriamfetol, discovered by SK Biopharmaceuticals and licensed out to Aerial BioPharma who then licensed out to Jazz Pharmaceuticals. Solriamfetol is a drug for exessive daytime sleepiness. It is sold in the US by Jazz under the brand name Sunosi.
SK Biopharmaceutical prides itself in having discovered the investigational molecule that went on to gain a nod from the FDA.
More important for the company is the second successful pipeline product cenobamate, an alternative drug for adults suffering partial-onset epileptic seizures. The drug was especially noted for having achieved complete removal of seizures in 28 percent of the patients who took the drug in clinical tests. The median rate of seizure frequency also significantly decreased 56 percent.
Cenobamate is independently distributed by SK Biopharmaceutical‘s US arm SK Life Science under the brand name Xcopri.
The proceeds from the IPO will be the ammunition for the company to carry out Xcopri’s marketing and sales in the US, as well as to develop other promising pipelines.
SK Biopharmaceuticals’ other pipelines in development encompass carisbamate, relenopride, SKL13865, SKL20540, SKL-PSY and SKL24741 which are for early childhood epilepsy, attention deficit disorder, schizophrenia and bipolar disorder.
SK Group Chairman Chey Tae-won had set his eyes on bio business since 2002, having declared to make bio one of the core pillars of SK‘s businesses by 2030.
Chey’s vision is a full value chain of pharmaceuticals business. In 2018, SK Holdings acquired US‘ Ampac Fine Chemicals, and consolidated it with SK Biotek in Korea, SK Biotek Ireland in Europe. The result is the SK Pharmteco, a contract manufacturing organization for pharmaceuticals.
SK Biopharmaeceuticals’ active pharmaceutical ingredients are manufactured by SK Biotek.
In the larger picture, the mega-blockbuster IPO is likely to empower SK Holdings as the parent entity owns 75 percent of the CNS disorder treatments company. The IPO deal may clear obstacles for a much-rumored governance shift within the SK Group, according to market watchers.
In January, prior to the COVID-19 shock, market watchers had mulled the possibility of a merger between SK Holdings and SK Telecom, to streamline its telecommunications and investment businesses, and also grant more freedom to its second-tier subsidiary SK hynix, the nation’s second-largest chipmaker.
SK Holdings owns 25 percent of SK Telecom. The telecom giant has a 20 percent stake in SK hynix.
By law, the ownership of a third-tier subsidiary is allowed only when the second-tier subsidiary acquires 100 percent of its shares, meaning that if SK hynix wants to acquire a company, it has to buy all its shares.
There remains a variable, however, which is SK Group Chairman’s divorce suit with ex-president’s Roh Tae-woo‘s daughter Roh Soh-yeong. Roh has requested Chey for a 42.3 percent of his SK Holdings shares worth 1 trillion won.
Chey currently owns 18.44 percent stake in SK Holdings, which is some 12.9 million shares.
SK Holdings declined to comment on the rumors of governance shift, but said that the SK Biopharmaceuticals‘ IPO was a hard-earned, due course of event.
By Lim Jeong-yeo, Jie Ye-eun (kaylalim@heraldcorp.com) (yeeun@heraldcorp.com)







![[KH Explains] How should Korea adjust its trade defenses against Chinese EVs?](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/15/20240415050562_0.jpg&u=20240415144419)










![[Today’s K-pop] Stray Kids to return soon: report](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/04/16/20240416050713_0.jpg&u=)