Korean companies raise bar on coronavirus test kits
KogeneBiotech, Seegene and PCL produce real-time PCR test kits that can detect infections in six hours
By Lim Jeong-yeoPublished : Feb. 25, 2020 - 15:48
Korean companies specializing in infection test kits are exporting their new real-time PCR COVID-19 assay kits to overseas destinations.
They include KogeneBiotech, Seegene and PCL.
With vaccine development for COVID-19 anticipated to take at least a year, the most realistic containment measure currently is to make a speedy diagnosis and follow up with effective quarantine, experts opine.
While the numbers of confirmed cases have shot up in Korea -- 977 as of Tuesday at 5:30 p.m. -- the country seemingly has the test kit situation under control, with pharmaceutical companies springing into action, according to industry watchers.
The only downside is that the tests cost 160,000 won ($132), and are only free for those showing symptoms within 14 days since visiting China, those showing symptoms within 14 days since coming into close contact with a confirmed patient or those who have a doctor’s opinion on having symptoms.
The test involves collection of nasal discharge from the upper airway using long cotton swabs and sputum induced from lower respiratory tract, which can be an uncomfortable experience, Korea’s Centers for Disease Control and Prevention said.
Meanwhile, the KCDC hotline for COVID-19 consultation -- 1339 -- has been inundated with calls leading to disruptions in service, as suspicious cases continue to increase.
Since the Middle East respiratory syndrome that hit Korea in 2015, the state agency had set up a protocol to facilitate faster detection of pathogens from patients with fever from an unidentified cause.
The KCDC introduced a real-time polymerase chain reaction test on Jan. 13, that can yield results in under six hours through amplification of targeted DNA. The previously employed method had required a day or two for detection.
They include KogeneBiotech, Seegene and PCL.
With vaccine development for COVID-19 anticipated to take at least a year, the most realistic containment measure currently is to make a speedy diagnosis and follow up with effective quarantine, experts opine.
While the numbers of confirmed cases have shot up in Korea -- 977 as of Tuesday at 5:30 p.m. -- the country seemingly has the test kit situation under control, with pharmaceutical companies springing into action, according to industry watchers.
The only downside is that the tests cost 160,000 won ($132), and are only free for those showing symptoms within 14 days since visiting China, those showing symptoms within 14 days since coming into close contact with a confirmed patient or those who have a doctor’s opinion on having symptoms.
The test involves collection of nasal discharge from the upper airway using long cotton swabs and sputum induced from lower respiratory tract, which can be an uncomfortable experience, Korea’s Centers for Disease Control and Prevention said.
Meanwhile, the KCDC hotline for COVID-19 consultation -- 1339 -- has been inundated with calls leading to disruptions in service, as suspicious cases continue to increase.
Since the Middle East respiratory syndrome that hit Korea in 2015, the state agency had set up a protocol to facilitate faster detection of pathogens from patients with fever from an unidentified cause.
The KCDC introduced a real-time polymerase chain reaction test on Jan. 13, that can yield results in under six hours through amplification of targeted DNA. The previously employed method had required a day or two for detection.

KogeneBiotech, the company that had collaborated with the KCDC for the test kit, said it followed the World Health Organization’s guideline. It was the first company to release its PowerChek 2019 nCoV Real-time PCR Kit to obtain emergency use approval and listing from the KCDC.
While generally such new test kits require a year to pass the Ministry of Food and Drug Safety’s regulations, in times of emergency the rules are temporarily softened to allow for a quicker response.
KogeneBiotech’s kit is being supplied to the KCDC and to over 50 hospitals in Korea designated to test suspected coronavirus patients. The company is also beginning to evaluate the test in China, Japan, Saudi Arabia, the United Arab Emirates, Iraq, Vietnam and Bahrain.
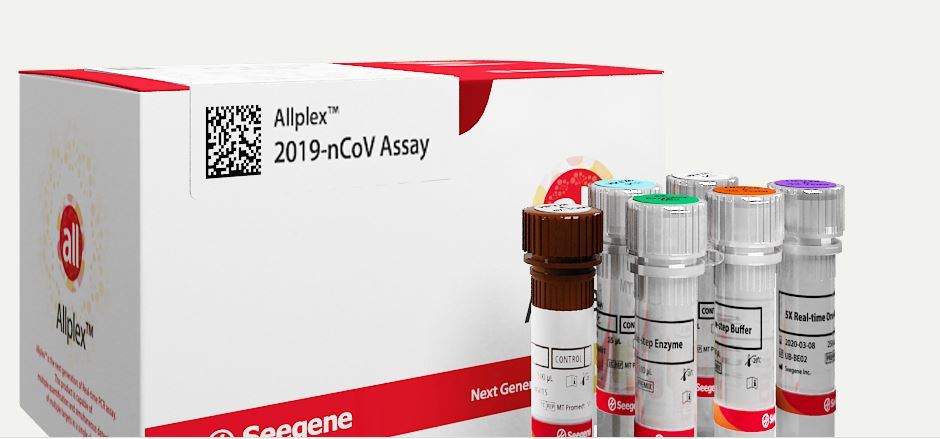
Seegene’s assay kits obtained European approval before Korean approval, and are being supplied domestically as well as to the company’s overseas entities in Italy and Germany.
Seegene’s AllplexTM 2019-nCoV assay kit does simultaneous detection and identification of three target genes specific to COVID-19.
The company also has offices in Dubai and Brazil, which are priority regions for its kit shipments.
Seegene has a daily manufacturing capacity of 50,000 assay kits at its facility in Jamsil, Seoul, and could double capacity if needed, a company official said.

PCL has collaborated with OliX Pharmaceutical in developing a real-time PCR kit. It has applied for approval with the KCDC, and after getting the green light will begin the process for export to China and India, a PCL official said.
By Lim Jeong-yeo (kaylalim@heraldcorp.com)











![[Today’s K-pop] BTS pop-up event to come to Seoul](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/17/20240417050734_0.jpg&u=)





![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)

