Kolon Life Science launches osteoarthritis drug Invossa in Korea
By Sohn Ji-youngPublished : Nov. 8, 2017 - 15:02
Kolon Life Science has begun selling Invossa, a novel cell-mediated therapy for degenerative arthritis, in South Korea, with the first patient injection taking place Wednesday, the company said.
Invossa is the world’s first gene therapy for treating osteoarthritis of the knee, in which the cartilage that cushions the joints wears away, causing the bones to rub together and induce pain.
A single injection of the drug delivers therapeutic relief for up to two years, according to the firm. It was developed by Kolon Group’s US-based bioparma subsidiary TissueGene, which went public on the Kosdaq this week.
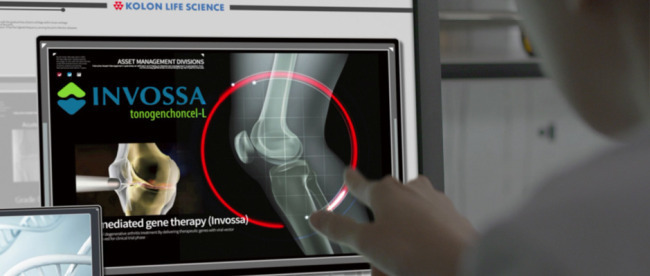
The drug was approved by Korea’s Ministry of Food and Drug Safety in July for patients with osteoarthritis in the knee who continue to experience pain even after receiving pain-relieving drugs or physical therapy for more than three months.
Seoul-based Kolon Life Science, which owns a 14.37 percent stake in TissueGene, is in charge of Invossa’s marketing and sales in Korea.
“We will work to maintain a smooth supply flow to ensure that Invossa can reach knee osteoarthritis patients everywhere including local neighborhood clinics and major hospitals,” said Kolon Life Science CEO Lee Woo-su in a statement.
Looking ahead, TissueGene is working to prove via clinical trials that the drug can help with tissue regeneration. The drug was approved only for pain relief and function improvement in Korea, limiting its therapeutic applications.
The Maryland-based drugmaker is looking to amass evidence of Invossa’s regenerative properties during phase 3 clinical trials slated to take place in the US next month. The firm is aiming to commercialize the drug by 2023.
The success of Invossa’s overseas trials are expected to determine the company’s corporate value and share price in the future. There is currently no treatment for degenerative osteoarthritis, only pain-relieving therapies, meaning a new drug with regenerative properties would be highly valued in the market.
By Sohn Ji-young (jys@heraldcorp.com)
Invossa is the world’s first gene therapy for treating osteoarthritis of the knee, in which the cartilage that cushions the joints wears away, causing the bones to rub together and induce pain.
A single injection of the drug delivers therapeutic relief for up to two years, according to the firm. It was developed by Kolon Group’s US-based bioparma subsidiary TissueGene, which went public on the Kosdaq this week.

The drug was approved by Korea’s Ministry of Food and Drug Safety in July for patients with osteoarthritis in the knee who continue to experience pain even after receiving pain-relieving drugs or physical therapy for more than three months.
Seoul-based Kolon Life Science, which owns a 14.37 percent stake in TissueGene, is in charge of Invossa’s marketing and sales in Korea.
“We will work to maintain a smooth supply flow to ensure that Invossa can reach knee osteoarthritis patients everywhere including local neighborhood clinics and major hospitals,” said Kolon Life Science CEO Lee Woo-su in a statement.
Looking ahead, TissueGene is working to prove via clinical trials that the drug can help with tissue regeneration. The drug was approved only for pain relief and function improvement in Korea, limiting its therapeutic applications.
The Maryland-based drugmaker is looking to amass evidence of Invossa’s regenerative properties during phase 3 clinical trials slated to take place in the US next month. The firm is aiming to commercialize the drug by 2023.
The success of Invossa’s overseas trials are expected to determine the company’s corporate value and share price in the future. There is currently no treatment for degenerative osteoarthritis, only pain-relieving therapies, meaning a new drug with regenerative properties would be highly valued in the market.
By Sohn Ji-young (jys@heraldcorp.com)







![[Graphic News] More Koreans say they plan long-distance trips this year](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/17/20240417050828_0.gif&u=)
![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=644&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)





![[From the Scene] Monks, Buddhists hail return of remains of Buddhas](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/19/20240419050617_0.jpg&u=20240419175937)

![[KH Explains] Hyundai's full hybrid edge to pay off amid slow transition to pure EVs](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=652&simg=/content/image/2024/04/18/20240418050645_0.jpg&u=20240419100350)

![[Today’s K-pop] Illit drops debut single remix](http://res.heraldm.com/phpwas/restmb_idxmake.php?idx=642&simg=/content/image/2024/04/19/20240419050612_0.jpg&u=)